COVID-19
Rapid Antigen Test Operating Instruction
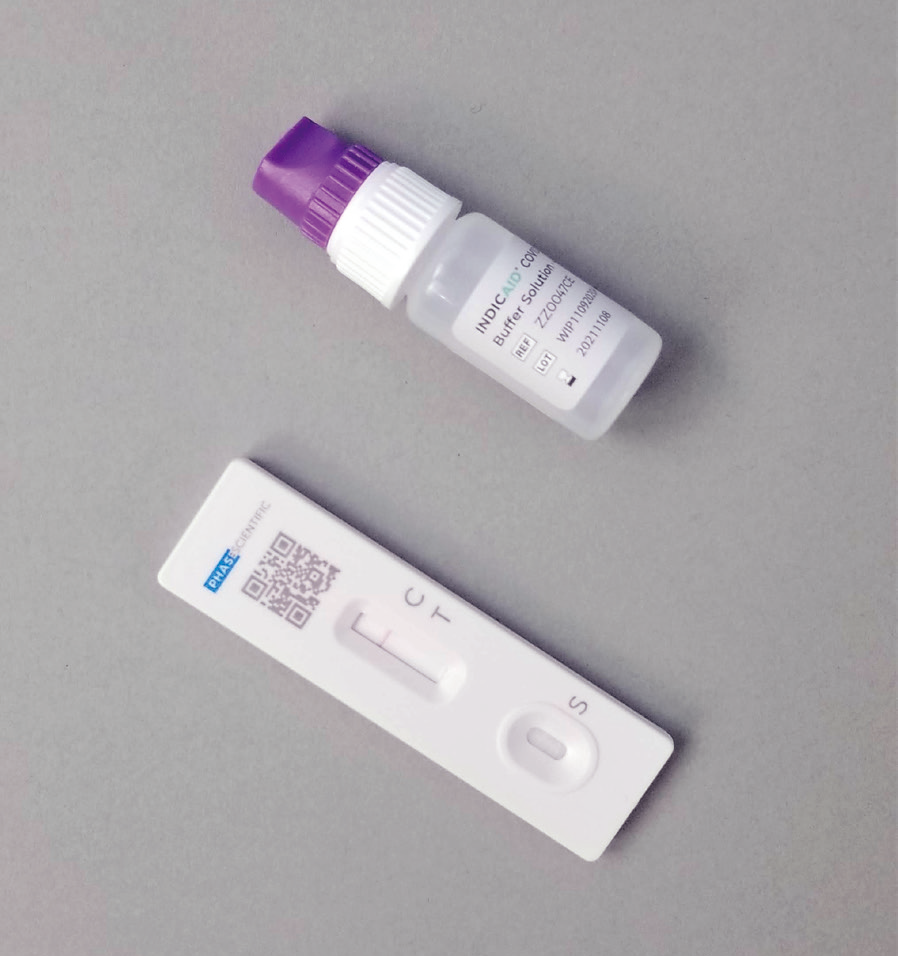

COVID-19 Rapid Antigen Detection Kit
INDICAID® COVID-19 Rapid Antigen Test (FDA EUA, CE) is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens in direct nasal swab samples.
Product Strengths
- Fast Results: Results can be obtained in just 20 minutes
- High Sensitivity: Clinically validated with overall sensitivity and specificity at 96% and 99% respectively
- Low cost: Testing is inexpensive
- Simple operation: No special equipment or facilities needed
- Easy to use: Anyone can use it anytime, anywhere
About Antigen Test
- What is the COVID-19 antigen?
An antigen is a molecule or molecular structure outside of a pathogen that, when detected in the human body, can trigger an immune response. Common antigens include simple intermediate metabolites such as carbohydrates, fats, hormones, and larger molecules including polysaccharides, phospholipids, nucleic acids, and proteins. - How does COVID-19 antigen tests work?
Antigens are present on SARS-CoV-2 viruses, which can be often be found in the respiratory tract during early stage infections. These antigens, when detected, serve as markers for disease exposure. - When should COVID-19 Antigen Tests be used?
As the SARS-CoV-2 virus multiplies, the levels of the virus rise, peak around 1 week, then fall off around 2 weeks. COVID-19 antigen tests can be used around 2-14 days after infection to detect the presence of COVID-19 antigens. - How accurate are COVID-19 Antigen Tests?
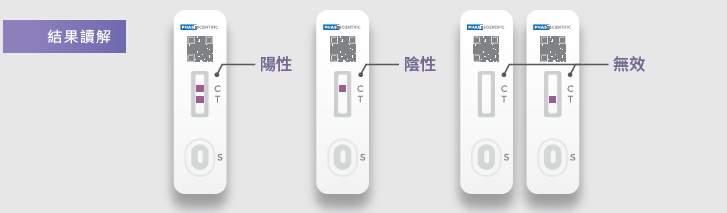
The accuracy of COVID-19 antigen tests are slightly lower than the gold standard PCR tests, however, its accuracy can reach 90% or above. The clinical specificity and sensitivity of the INDICAID COVID-19 Rapid Antigen Test reaches up to 99% and 96% respectively. - Who is COVID-19 antigen testing suitable for?
Antigen testing methods are simple, fast, inexpensive, and can be used in healthcare facilities and at home. It is particularly suitable for people with high incidence or frequent exposure to high-risk environments, and continuous testing (at least once a week) is carried out as a large-scale population screening. - Do COVID-19 antigen testing and PCR testing need to be done simultaneously?
If the antigen test is positive, a nucleic acid test is required to confirm the infection with COVID-19, and if the antigen test is negative, but has recently been in a viral environment or has been exposed to high-risk groups, nucleic acid testing is recommended to confirm it. - What should I do if the test result is positive?
If you tested positive for COVID-19 antigens, we recommend doing another test with a brand new Rapid antigen test kit. If both tests reveal positive results, please contact the support hotline provided by PHASE Scientific at 9014 6321 for the arrangement of a free PCR test.

